Unique Device Identification#
Overview#
To simplify the tracking of medical products (in the case of recalls especially) and to prevent counterfeit goods from being sold, manufacturers must assign a UDI to every product and its packaging prior to the item entering the market.
With the UDI functionality that can be integrated into Microsoft Dynamics 365 Business Central1, you can maintain the information you need to meet Unique Device Identification rules and prepare GUDID and EUDAMED reports.
Note
You need a separate license to use this functionality.
Abbreviations#
| Acronyms / Initialisms | Description |
|---|---|
| DUNS | Data Universal Numbering System |
| EUDAMED | European Database on Medical Devices |
| GUDID | Global Unique Device Identification Database |
| GUDID Data Elements Reference Table | (List of UDI data elements as published by the FDA) |
| UDI | Unique Device Identification |
| UDI Data Dictionary | (List of UDI data elements as published by the European Commission) |
| XML | Extensible Markup Language |
| XSD | XML Scheme Definition |
UDI User Setup#
To configure users’ individual access rights to UDI data:
- Choose the Search icon, enter UDI Setup, and then choose the related link.
- Enter or select a user and fill in the fields as necessary.
Set up EUDAMED#
UDI Setup#
To set up the UDI-EUDAMED interface:
- Choose the Search icon, enter UDI Setup, and then choose the related link.
- On the General and EUDAMED FastTabs, fill in the fields described in the following table.
| Field | Description |
|---|---|
| Default Issuing Entity / Agency Code | Specify the default issuing entity. |
| Default Manufacturer SRN | Specify the manufacturer’s default SRN. |
Actions#
New DI#
Creates a new DI, such as a Basic DI, a UDI-DI, or a Secondary DI.
Related – Base Data#
Contains the functions needed to maintain basic EUDAMED data, as described in the European Commission’s UDI Data Dictionary. For more information on a specific field function, see the following table.
| Function | Description |
|---|---|
| CMR Categories | Specifies if the device contains a CMR (carcinogenic, mutagenic, or toxic to reproduction) substance. |
| Clinical Size Types | Specifies the type of clinical size associated with the device. Sizes can be identified by their type (such as length or width), precision (single value, range, or text), or unit of measure. |
| Device Certificate Types | Specifies the type of product certificate assigned to the device. This type is part of the data that must be provided when you register products that need to be certified. |
| Issuing Entities | Specifies the issuing entities tasked by the European Commission with creating EUDAMED DI codes (Basic UDI-DIs, UDI-DIs, Unit of Use DIs, Secondary DIs, and Package Level DIs). |
| EMDN Codes | Specifies EMDN nomenclature code(s) associated with the UDI-DI. |
| Measure Units | Specifies the unit used for the clinical size associated with the device. Sizes can be identified by their type (such as length or width), precision (single value, range, or text), or unit of measure. |
| Multi-Component Devices | Specifies if the Basic UDI-DI refers to a system that is a device itself, a procedure pack that is a device itself in accordance with Article 22(4) MDR, or a kit. This field is applicable only to standard devices, not systems or procedure packs. The Kit option is also only applicable to IVDR devices and systems that are devices themselves. In the case of procedure packs, this field applies only to MDR devices. |
| Non-Medical Device Types (Annex XVI) | References the intended non-medical purpose of the device (UDI-DI) as described in Annex XVI of the MDR regulation. |
| Notified Bodies | References the organization that issued the product certificate for the device. The organization’s contact details are part of the data that must be provided when you register products that need to be certified. |
| Risk Classes | Specifies the risk class of the device that is assigned to the Basic UDI-DI. What risk class the device is put in depends on the legislation applicable to it. |
| Special Devices | Specifies if the device is of a special type and if so, the type thereof. You cannot use this function if the device is a standard device that has the options of System or Procedure Pack (devices themselves) or is a system or procedure pack. |
| Storage and Handling | References the storage and handling conditions associated with the device (UDI-DI). |
| Types of Substances | Specifies the type of medicinal substances contained in the device. This includes substances understood as a medicinal product or as a medicinal product derived from human blood or plasma. |
| Warning Values | Specifies critical warnings or contra-indications linked to the UDI-DI. |
Related – UDI#
| Function | Description |
|---|---|
| DI List | Shows a list of all DIs, such as Basic DIs, UDI-DIs, and Secondary DIs, including those that have already been assigned. |
| Product Designers | Specifies the product designer, that is, the company who originally manufactured or designed the device. Also provides data on manufacturers and designers not available in EUDAMED. |
| Basic UDI-DI List | Shows a list of all Basic UDI-DIs, including those already assigned. |
| Change Log | Lists the changes made to UDI information. |
DI List#
This list stores all DIs available in the system, including DIs already in use and those not in use but acquired from issuing entities. The following chart shows the regulations that certain types of EUDAMED data are subject to.
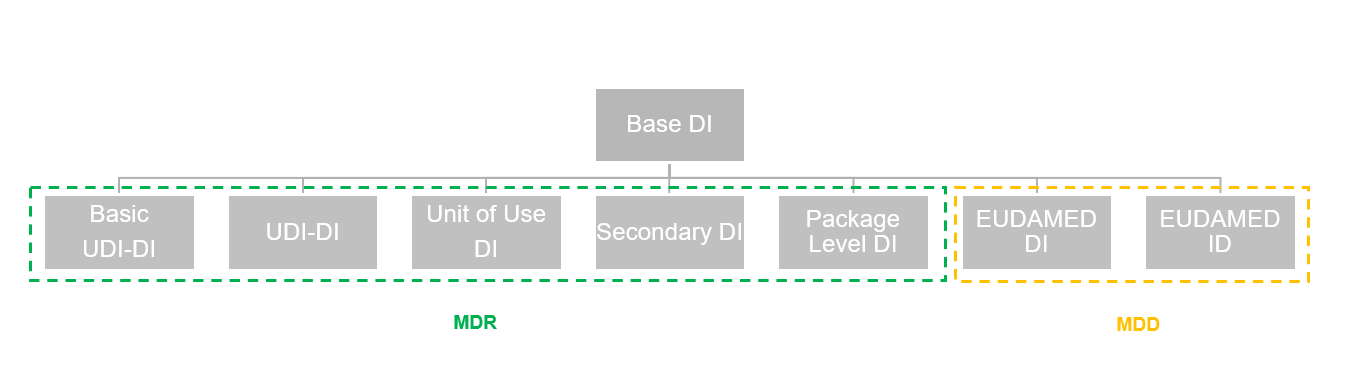
The fields found on each line of the list are described in the following table.
| Field | Description |
|---|---|
| Code | Specifies the relevant UDI-DI, such as GTIN or HIBC. |
| Issuing Entity / Agency Code | Specifies the issuing entity, such as GS1. |
| Description | Can be used for a more detailed description of the record. This description will not be copied to EUDAMED (or GUDID). |
| DI Type | Specifies the type of DI: • None (placeholder for acquired but not yet assigned DIs) • Basic UDI-DI • UDI-DI • Unit of Use DI • Secondary DI • Package Level DI • EUDAMED DI • UDI-DI (Legacy) |
| Used in EUDAMED | Specifies whether the DI is part of a EUDAMED record. |
| Used in GUDID | Specifies whether the DI is part of a GUDID record. |
Actions#
Based on the type of DI, you can choose one of the following functions on the ribbon:
| Function | Description |
|---|---|
| Create EUDAMED DI | Creates a new EUDAMED record with a Basic UDI-DI, UDI-DI, or EUDAMED DI. |
| New | Inserts a new line on the page. |
| Open DI Card | Opens the EUDAMED record. |
| Open DI Versions | Opens a list of all versions of the DI. |
| Open Item Relations | Opens the item relations specified for the record. |
| Audit Trail | Opens the change log for the record. |
Basic UDI Card#
You enter Basic UDI-DI data on the Basic UDI-DI Card. To enter new data:
- On the DI List, select a DI record of type Basic UDI-DI.
- Choose Open DI Card on the ribbon. To enter data that you previously used in GUDID or haven’t yet used at all, choose Create EUDAMED DI.
- Fill in the fields according to the UDI Data Dictionary. Fields that aren’t part of the dictionary are listed in the following.
General#
| Field | Description |
|---|---|
| Description | Specifies a text to describe the Basic UDI-DI. |
| Released | Specifies whether this version of the EUDAMED record has been released. |
| Active | Specifies whether this version is being or has been the last one edited. |
| Internal Version | Specifies the version of the Basic UDI-DI. |
Actions#
| Function | Description |
|---|---|
| Create New Version | Creates a new version of the Basic UDI-DI record. |
| Release Version | Releases the current Basic UDI-DI version. |
| Open DI Versions | Opens a list of all versions of the DI. |
| Audit Trail | Opens the change log for the record. |
UDI-DI card#
You enter UDI-DI data on the UDI-DI Card. To enter new data:
- On the DI List, select a DI record of type UDI-DI.
- Choose Open DI Card on the ribbon. To enter data that you previously used in GUDID or haven’t yet used at all, choose Create EUDAMED DI.
- Fill in the fields according to the UDI Data Dictionary. Fields that aren’t part of the dictionary are listed in the following.
General#
| Field | Description |
|---|---|
| Description | Specifies a text to describe the UDI-DI. |
| Released | Specifies whether this version of the EUDAMED record has been released. |
| Active | Specifies whether this version is being or has been the last one edited. |
| Internal Version | Specifies the version of the UDI-DI. |
Actions#
| Function | Description |
|---|---|
| Create New Version | Creates a new version of the UDI-DI record. |
| Release Version | Releases the current UDI-DI version. |
| Open DI Versions | Opens a list of all versions of the DI. |
| Open Item Relations | Specifies the relation of the UDI-DI to an item or its variant and unit of measure. |
| Audit Trail | Opens the change log for the record. |
Actions - Functions#
| Function | Description |
|---|---|
| Copy UDI Data | Copies base data from another UDI record. |
| Delete Basic UDI | Deletes the assigned Basic UDI. |
| Delete Secondary UDI | Deletes the assigned Secondary UDI. |
| Delete Unit of Use DI | Deletes the assigned Unit of Use DI. |
| Delete Direct Marking DI | Deletes the assigned Direct Marking DI. |
| Delete Legacy Device | Deletes the assigned Legacy Device. |
| Delete Product Designer Link | Deletes the link to the Product Designer. |
Legacy Device card#
You enter EUDAMED DI data on the Legacy Device card. To enter new data:
- On the DI List, select a DI record of type EUDAMED DI.
- Choose Open DI Card on the ribbon. To enter data that you previously used in GUDID or haven’t yet used at all, choose Create EUDAMED DI.
- Fill in the fields according to the UDI Data Dictionary. Fields that aren’t part of the dictionary are listed in the following.
Basic UDI#
| Field | Description |
|---|---|
| Description | Specifies a text to describe the DI. |
| Released | Specifies whether this version of the EUDAMED record has been released. |
| Active | Specifies whether this version is being or has been the one last edited. |
| Internal Version | Specifies the version of the DI. |
Actions#
| Function | Description |
|---|---|
| Create New Version | Creates a new version of the EUDAMED DI record. |
| Release Version | Releases the current version. |
| Open DI Versions | Opens a list of all versions of the DI. |
| Open Item Relations | Specifies the relation of a EUDAMED DI to an item or its variant and unit of measure. |
| Audit Trail | Opens the change log for the record. |
Actions - Functions#
| Function | Description |
|---|---|
| Copy from Legacy | Copies base data from another UDI record. |
| Delete Secondary UDI | Deletes the assigned Secondary UDI. |
| Delete Unit of Use DI | Deletes the assigned Unit of Use DI. |
| Delete Direct Marking DI | Deletes the assigned Direct Marking DI. |
| Delete Product Designer Link | Deletes the link to the product designer. |
Set up GUDID#
UDI Setup#
To set up the UDI-GUDID interface:
- Choose the Search icon, enter UDI Setup, and then choose the related link.
- On the General and GUDID FastTabs, fill in the fields described in the following table.
| Field | Description |
|---|---|
| Default Issuing Entity /Agency Code | Specify the default issuing agency. |
| DUNS Number | Specify the DUNS number that has been assigned by the FDA to identify the manufacturer. The number is used on documents and for reporting data to the agency. |
| Export Despite XSD Fail | Specifies if an XML export is possible despite failed XSD validation. |
| GUDID XSD Validation | Specifies if XSD validation is active (requires an XSD file). |
| GUDID XSD | Specifies if an XSD file has been stored for validation during the XML export process. |
Actions#
| Function | Description |
|---|---|
| New DI | Creates a new DI, such as a Basic DI, a UDI-DI, or a Secondary DI. |
| Import GUDID XSD | Imports a GUDID XSD file. |
| Delete GUDID XSD | Deletes the stored GUDID XSD file. |
Related – Base Data#
Contains the functions needed to maintain basic GUDID data, as described in the FDA UDI system. For more information on a specific field function, see the following table.
| Function | Description |
|---|---|
| Units of Measure | Specifies units of measure used for clinically relevant sizes, as well as storage and handling conditions. |
| FDA Product Codes | Specifies devices based on FDA categories. |
| GMDN Codes / FDA Preferred Term Codes | Specifies GMDN or Preferred Term (PT) codes. These are unique five-digit codes used to identify common device types. PT codes are assigned to medical devices and related health care products for the purposes of grouping and categorization. |
| Clinically Relevant Sizes | Specifies the dimension types for the clinically relevant measurement of the medical device. |
| Submission Types | Indicates the type of FDA premarket submission. This includes 510(k), De Novo, PMA, PDP, HDE, BLA, ANDA, and NDA. |
| Storage Handling Conditions | Indicates the storage and handling requirements for the device, such as temperature, humidity, and atmospheric pressure needs. |
| Sterilization Methods | Indicates the methods of sterilization that can be used for the device prior to use on a patient. |
DI List#
This list stores all DIs in the system. The DIs relevant to the GUDID database are shown in the following chart.
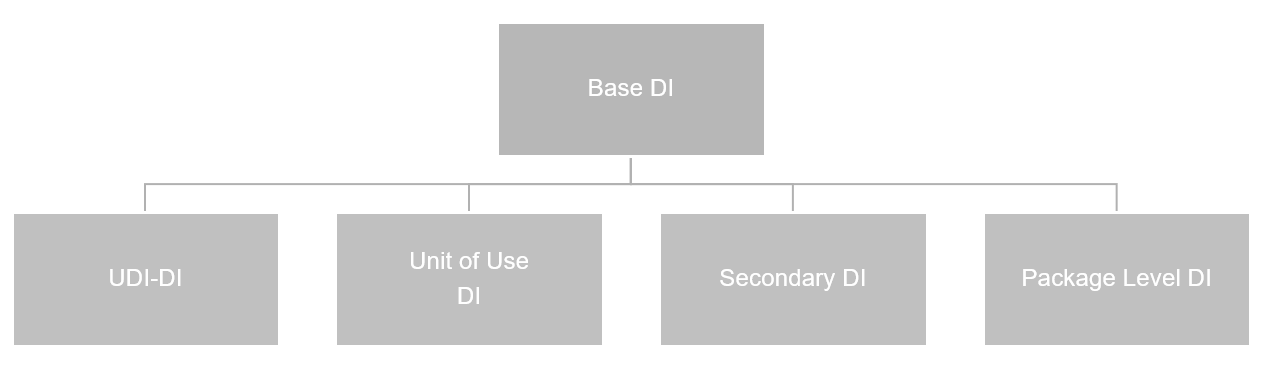
You set up GUDID data in the same way as you do EUDAMED information. For more details, see here.
-
Microsoft, Microsoft Dynamics, and Microsoft Dynamics 365 are trademarks of the Microsoft group of companies. ↩